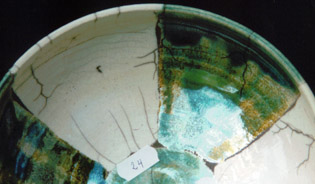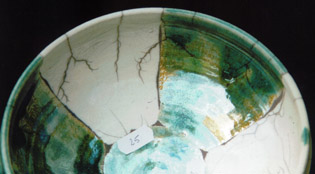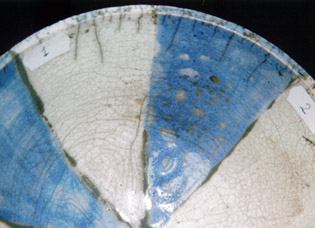
serie 1 - 4
1) The closest approximation of Gerstley borate with the frittes 3221/1451.
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 46 | .12 | .12 | .24 | .36 | ||
| Withing | 100 | 21 | .21 | .21 | ||||
| Dolomite | 184 | 29 | .16 | .16 | .16 | |||
| Fritte 3221 | 125 | 44 | .35 | .35 | .35 | |||
| Total | .12 | .16 | .72 | .59 | .36 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |
This glaze gives gas-bubbles, clearly visible in the picture with the blue colour. Crackles are not developed very well.
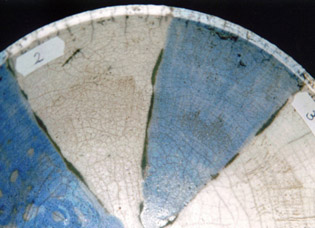
2) The closest approximation of Gerstley borate with the fritte 1451 and colemanite, the other materials are the same as 1).
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 46 | .12 | .12 | .24 | .36 | ||
| Withing | 100 | 32 | .32 | .32 | ||||
| Dolomite | 184 | 29 | .16 | .16 | .16 | |||
| Colemanite | 411 | 49 | .12 | .24 | .36 | |||
| Total | .12 | .16 | .72 | .60 | .36 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |
This glaze hasn’t melted well, the blue colour is more dull.

3)
The materials were chosen in such way that there isn’t any forming of CO2, as in the case of using whiting or dolomite.
Colomanite is chosen as B2O3-source.
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 46 | .12 | .12 | .24 | .36 | ||
| Talc | 379 | 19 | .05 | .15 | .20 | |||
| Wollastonite | 116 | 55 | .47 | .47 | .47 | |||
| Colemanite | 411 | 49 | .12 | .24 | .36 | |||
| Total | .12 | .15 | .71 | .60 | 1.03 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |
The two new materials introduce more SiO2 and the glaze gives a poor crackle and melts bad.
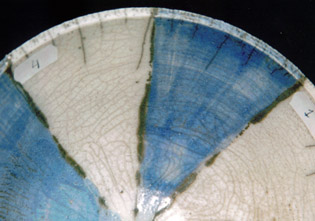
4)
Same materials as 3) with more colemanite to get a lower melting
temperature.
(As B2O3 source colomanite is chosen. )
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 46 | .12 | .12 | .24 | .36 | ||
| Talc | 379 | 19 | .05 | .15 | .20 | |||
| Wollastonite | 116 | 37 | .33 | .33 | .33 | |||
| Colemanite | 411 | 82 | .20 | .40 | .60 | |||
| Total | .12 | .15 | .73 | .84 | .89 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |
The larger amount of colemanite gives this glaze a better melting behaviour.
In the series this recipe gives the best results.

serie 5 - 10
In the series 5-7 the content of fritte 3221 has increased and because this is a Ca borate we have to lower the content
of wallastonite at the same time . (The content of Na2O, MgO, CaO is always the same).
The content of SiO2 decreases because the Wollastonite content decreases
5) Increase of the B2O3 content with fritte 3221 as source. (B2O3=.64 mol)
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 46 | .12 | .12 | .24 | .36 | ||
| Talc | 379 | 19 | .05 | .15 | .20 | |||
| Wollastonite | 116 | 35 | .30 | .30 | .30 | |||
| Fritte 3221 | 125 | 50 | .40 | .40 | .40 | |||
| Totaal | .12 | .15 | .70 | .64 | .86 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |

6) Increase of the B2O3 content with fritte 3221 as source. (B2O3=.79 mol)
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 46 | .12 | .12 | .24 | .36 | ||
| Talc | 379 | 19 | .05 | .15 | .20 | |||
| Wollastonite | 116 | 18 | .15 | .15 | .15 | |||
| Fritte 3221 | 125 | 68 | .55 | .55 | .55 | |||
| Totaal | .12 | .15 | .70 | .79 | .71 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |

7) Increase of the B2O3 content with fritte 3221 as source. (B2O3=.97 mol)
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 46 | .12 | .12 | .24 | .36 | ||
| Talc | 379 | 19 | .05 | .15 | .20 | |||
| Wollastonite | 116 | |||||||
| Fritte 3221 | 125 | 91 | .73 | .73 | .73 | |||
| Totaal | .12 | .15 | .73 | .97 | .56 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |
Number 7 in the series gives the best result.Also here we can see that a higher content of B2O3 gives beter results.

In this serie the B2O3 content has increased by Colemanite (and not by the fritte 3221).
The content of Na2O,MgO,CaO is always the same
8) Increase of the B2O3 content with Colemanite as source. (B2O3=.99 mol)
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 46 | .12 | .12 | .24 | .36 | ||
| Talk | 379 | 19 | .05 | .15 | .20 | |||
| Wollastoniet | 116 | 23 | .20 | .20 | .20 | |||
| Colemaniet | 411 | 102 | .25 | .50 | .75 | |||
| Totaal | .12 | .15 | .70 | .99 | .76 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |

9) Increase of the B2O3 content with Colemanite as source. (B2O3=1.14 mol)
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 46 | .12 | .12 | .24 | .36 | ||
| Talk | 379 | 19 | .05 | .15 | .20 | |||
| Wollastoniet | 116 | 12 | .10 | .10 | .10 | |||
| Colemaniet | 411 | 123 | .30 | .60 | .90 | |||
| Totaal | .12 | .15 | .70 | 1.14 | .66 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |

10) Increase of the B2O3 content with Colemanite as source. (B2O3=1.29 mol)
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 46 | .12 | .12 | .24 | .36 | ||
| Talk | 379 | 19 | .05 | .15 | .20 | |||
| Wollastoniet | 116 | |||||||
| Colemaniet | 411 | 144 | .35 | .70 | 1.05 | |||
| Totaal | .12 | .15 | .70 | 1.29 | .56 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |
In the series 5-7 the content of colemanite has been increased, at the same time the content of wallastonite has lowered to zero. (The content of Na2O, MgO, CaO is always the same).
By increasing the content of colemanite the amount of gas-bubbles is also increasing as you can see by the pinholes in the glaze.
Number 7 in the series gives the best result.

serie 11 - 13
Study of the influence of fritte 1451 (and thus of the Na2O content)
11) NaO=.06 mol%
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | 23 | .06 | .06 | .12 | .18 | ||
| Talc | 379 | 19 | .05 | .15 | .20 | |||
| Colemanite | 411 | 164 | .40 | .80 | 1.20 | |||
| Total | .06 | .15 | .80 | 1.32 | .38 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |

12) Na2O=0 mol
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Fritte 1451 | 381 | |||||||
| Talc | 379 | 19 | .05 | .15 | .20 | |||
| Colemanite | 411 | 172 | .42 | .84 | 1.26 | |||
| Total | .15 | .84 | 1.26 | .20 | ||||
| GB RR | .12 | .16 | .72 | .59 | .33 |

13) Na2O=.13 mol
Decrease of the content of fritte 1451 to zero, but now fritte 1510 as a source of Na2O.
( .13 mol Na2O ,the same as in exp.10)
This experiment is carried out to see if the pinholes -by using colemanite - were caused by the low melting temperature
of fritte 1451.
(The low meltingpoint of one of the components can have an effect on the evaporation of water
normaly present in colemanite - 2CaO.3B2O3.2H2O -)
(The result of the experiment 12) wasn’t known, otherwise this result would make
clear that the melting behaviour of fritte 1451 didn’t have any influence).
| material | molweight | weight | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 | |
| Fritte 1510 | 198 | 40 | .2 | .13 | .02 | .04 | .42 | ||
| Talk | 379 | 19 | .05 | .15 | .20 | ||||
| Colemaniet | 411 | 144 | .35 | .70 | 1.05 | ||||
| Totaal | .13 | .15 | .72 | .04 | 1.05 | .62 | |||
| GB RR | .12 | .16 | .72 | .59 | .33 |
The glazes are rather bad with in general many pinholes , there are a lot of gas-bubbles during the firing.

serie 14 - 16
In experiment 12) the Na2O content has been lowered to zero and this has no
influence on the results, as a consequence also the amount of MgO has been decreased to see what happens.
This is done with Colemanite as well with fritte 3221 as a B2O3 source.
14) content of talc in recipe 12) is now 0.
| materiaal | molgew | gewicht | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Talk | 379 | |||||||
| Colemaniet | 411 | 206 | .50 | 1.0 | 1.50 | |||
| Fritte 3221 | 125 | |||||||
| Totaal | 1.0 | 1.5 | ||||||
| GB RR | .12 | .16 | .72 | .59 | .33 |

15) “same” recipe of 12) now with fritte 3221 as B2O3 source, colemanite=0
| materiaal | molgew | gewicht | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Talk | 379 | 19 | .05 | .15 | .20 | |||
| Colemaniet | 411 | |||||||
| Fritte 3221 | 125 | 106 | .85 | .85 | .85 | |||
| Totaal | .15 | .85 | .85 | .20 | ||||
| GB RR | .12 | .16 | .72 | .59 | .33 |
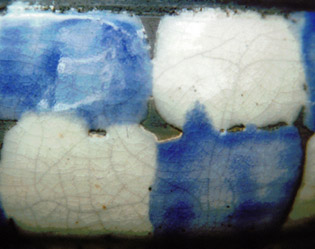
16) same as 15) now without talc.
| materiaal | molgew | gewicht | Mol% | Na2O | MgO | CaO | B2O3 | SiO2 |
| Talk | 379 | |||||||
| Colemaniet | 411 | |||||||
| Fritte 3221 | 125 | 125 | 1.0 | 1.0 | 1.0 | |||
| Totaal | 1.0 | 1.0 | ||||||
| GB RR | .12 | .16 | .72 | .59 | .33 |
The result of the experiments 15-16 are the best, there isn’t any gasformation during the firing and that’s the reason that there aren’t any pinholes in the glaze.
Experiment 14, with only colemanite, gives a lot of gas and pinholes and a rather bad crackle.
The use of colemanite in Raku glazes isn’t a good choice! (although the price of the material is low.) A special addition of Na2O, MgO, CaO isn’t necessary when we use nepheline syanite !!
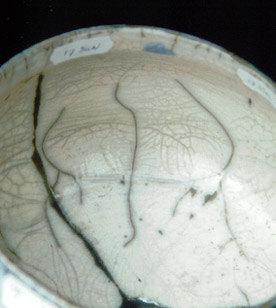
serie 17 - 19
Now that we know that only with fritte 3221 and nepheline syanite it is possible to make a good glaze we want to examine
if the ratio between the two materials (till now always in weight-ratio 1:1) has a pronounced effect on the results.
Also the thickness of the layer is a part of the experiment
17) ratio 3221 :NS = 8 : 12 (thin/thick layer)
Thin layer gives better crackles.

18) ratio 3221 :NS = 10 : 10 (thin/thick layer)
Thin layer gives better crackles.
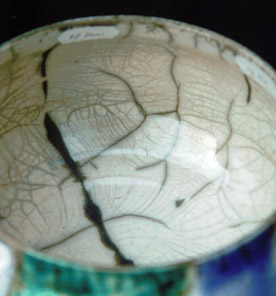
19) ratio 3221 :NS = 12 : 8 (thin/thick layer)
Thin layer gives better crackles.
Conclusion: thin layers give a better crackle than thick layers.
There is a small difference in the ratio of the fritte 3221 and nepheline syanite, experiment 17/18 is a little better
than 19 and fortunately it is cheaper too!!
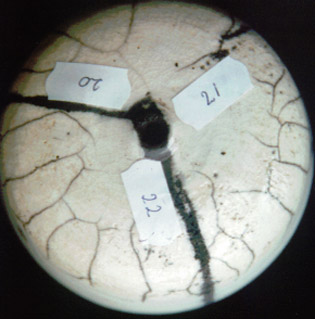
serie 20 - 22
The recipe found up to now is good and simple, however it’s difficult to spread and so the possibility to apply the glaze
with a brush isn’t optimum.
In this series ball clay is added to improve the spreadability.
| material | exp. 20 | exp. 21 | exp. 22 |
| fritte 3221 | 10 | 10 | 10 |
| nepheline syanite | 10 | 9 | 8 |
| ball clay | 1 | 2 |
In the forming of crackles there is no big difference between the recipes.
In experiment 22 the spreadability (and also the strength of the layer) is comparable with Gerstley borate
(which is excellent to apply with a brush).
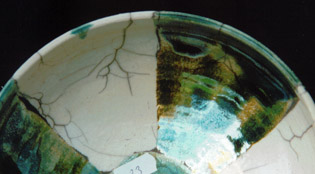
Serie 23 - 25
In an attempt to make the recipe more complicated (you are an alchemist or not.) I have played with some additions of Na2O
in the form of salt (NaCl) or soda (NaHCO3.H2O).
From top to bottom:
exp.23) same recepee as 22)
exp.24) 1 gram addition of salt (naCl)
exp 25) 1 gram addition of soda (NaHCO3.H2O)
There wasn’t any difference in results, pity? No! because simplicity is also beautiful.